
Basics Chemistry about Endopeel
The main ingredients to realize endopeel technics are :
- phenic acid
- peanuts oil acid
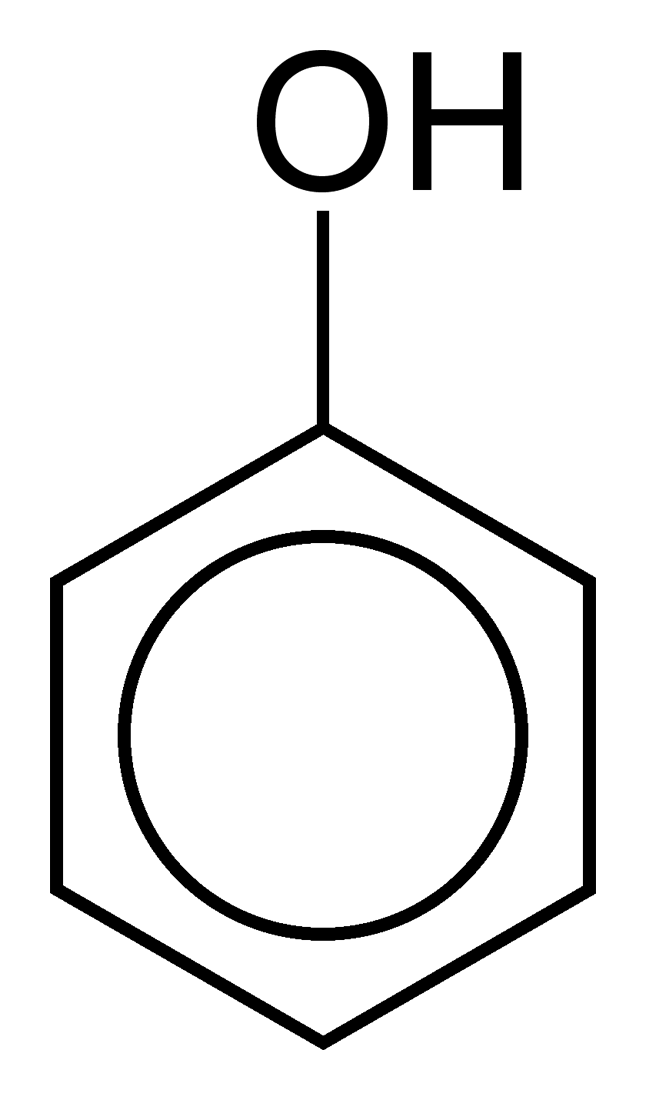
Phenic Acid
Phenic Acid ,Carbolic Acid and Phenol have been considered similar for most organic chemistry engineers as medical doctors, pharmacists ...
In fact Endopeels carbolic acid as endopeels phenic acid are weak acids with pKa <7 and phenol has a pKa>7 .
That means that carbolic acid and phenol like on the image are same in organic chemistry formula development, but they are completely different concerning endopeel in general chemistry and physico chemistry.
There are 3 main differences :
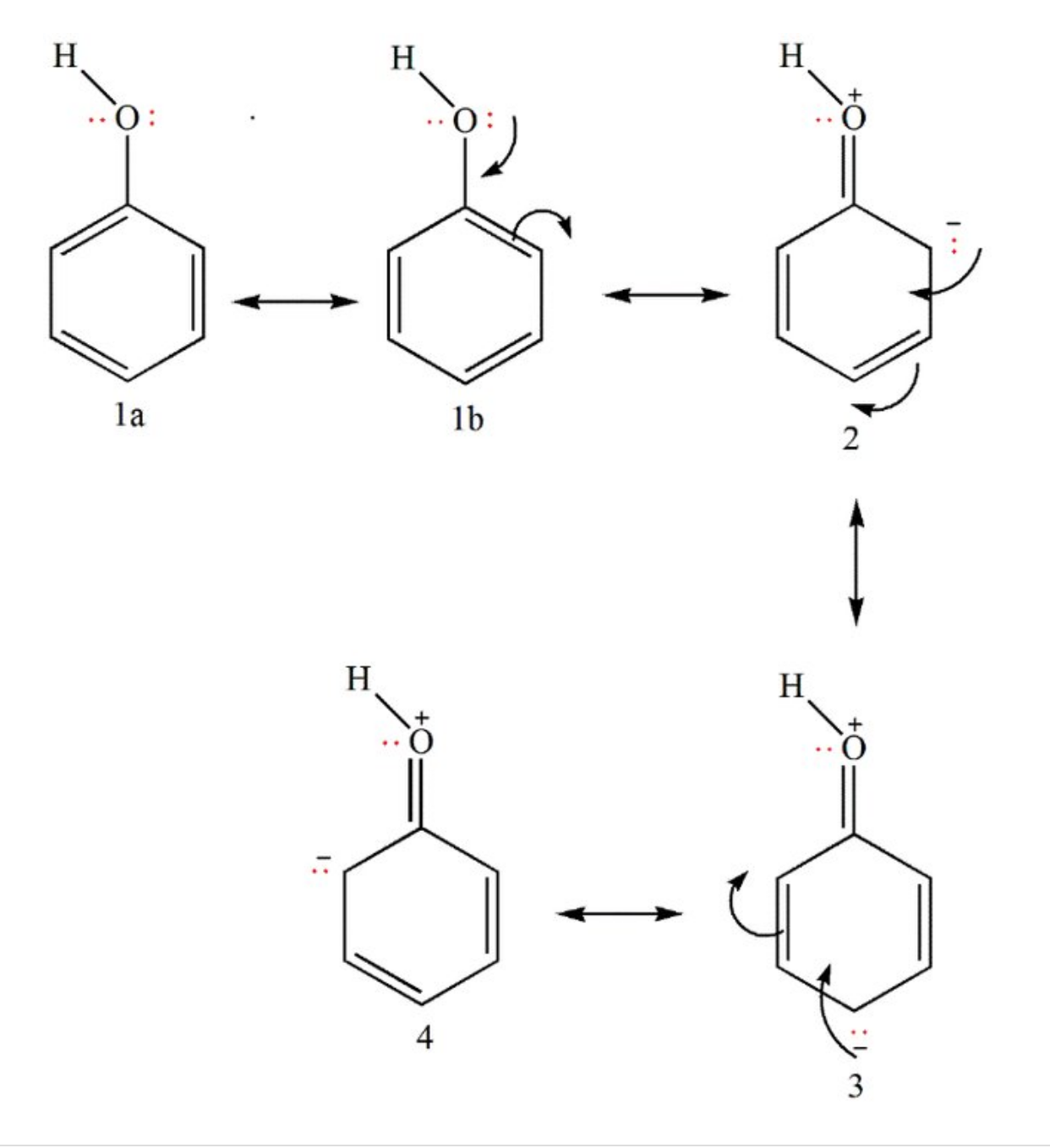
- Resonance stabilization of the phenoxide anion by the aromatic ring.In this way, the negative charge on oxygen is shared by the ortho and para carbon atoms. That is why endopeels carbolic acid is used instead of phenol for endopeel techniques ( which lead to medical liftings obtained by chemical myoplasty, myopexy and myotension)
- Increased acidity in the result of orbital overlap between the oxygen's lone pairs and the aromatic system.
- The dominant effect is the induction from the sp2 hybridized carbons;the comparatively more powerful inductive withdrawal of electron density
Resonance Structures for Phenol
1-Dewandre L., Tenenbaum A.. Chemical Peels of Drs. Mark G. Rubin and Rebecca Tung 2 nd Edition. Imprint: Saunders. Chapter 1: Chemistry of Peelings and Hypothesis of the Mechanism of Action ISBN: 978-1-4377-1924-6. Copyright: 2011

Arachidonic Acid

Equation of Henderson Hasselbach
Legend :
- HA represents the molecular form of the weak acid with where H is the atom of hydrogen
- A- represents the anion and H+ the proton, which in solution leads to the ion hydronium H30+
- Ka is the constant of acidity .
The pKa of endopeel phenic acid is 6.65 vs the pKa of phenol is 9.95.
Definitions of important terms in stereochemistry.
-
Stereoisomers
Have similar molecular formula but differ in spatial orientation of their atoms.
-
Enantiomers
are 2 stereoisomers with non-superimposable mirror images.
-
Distomer
Enantiomer with lower biological activity.
-
Eutomer
Eniantomer with higher biological activity
-
Racemate
A mixture of equal amounts of enantiomers.
-
Chiral Switch
A rasemate switch to single enantiomer.
-
Epimerization
Bioinversion of enantiomers.
-
(+) Optical Isomer
Rotate the light plane clockwise (dextrorotary).
-
(-) Optical Isomer
Rotate the light plane counter-clockwise (levorotary).
-
(R) and (L)
According to enantiomer configurations which are right or -left handed.
The chirality of Phenols
Phenols exhibit chirality within their molecules.
This chirality is due to the absence of planar and axial symmetry in the phenol molecule.
Scientific Definition

Ex :Left and right hands are mirror images but arent superimposable.
Chirality For Dummies

Hitler was a criminal nazi and Chaplin was a comic Jew
Interactions Implications for the Tissues
Molecule fits Receptor Site

Molecule doesnt fit Receptor Site

Conclusion

- The enantiomers of phenol are similar by their formula but their shape is different .
- Like a key in a lock,only the chirally correct form ( here the endopeels phenic acid) can interact with the appropriate cell receptor and trigger the right cellular response.
- The ,,wrong,, form of the phenol can collect on the skin s surface and can cause necrosis and big edema.
Ignorance & Prejudges
Einstein used to say that it was easier to break an atome than to break prejudges.
We do not have the right to open our mouth if our mind is close.
Courteline :
in french :
Passer pour un idiot aux yeux d un imbécile est un plaisir de fin gourmet.
Translated into english:
Look like an idiot in the eyes of a fool is a fun foodie

Albert Einstein


